
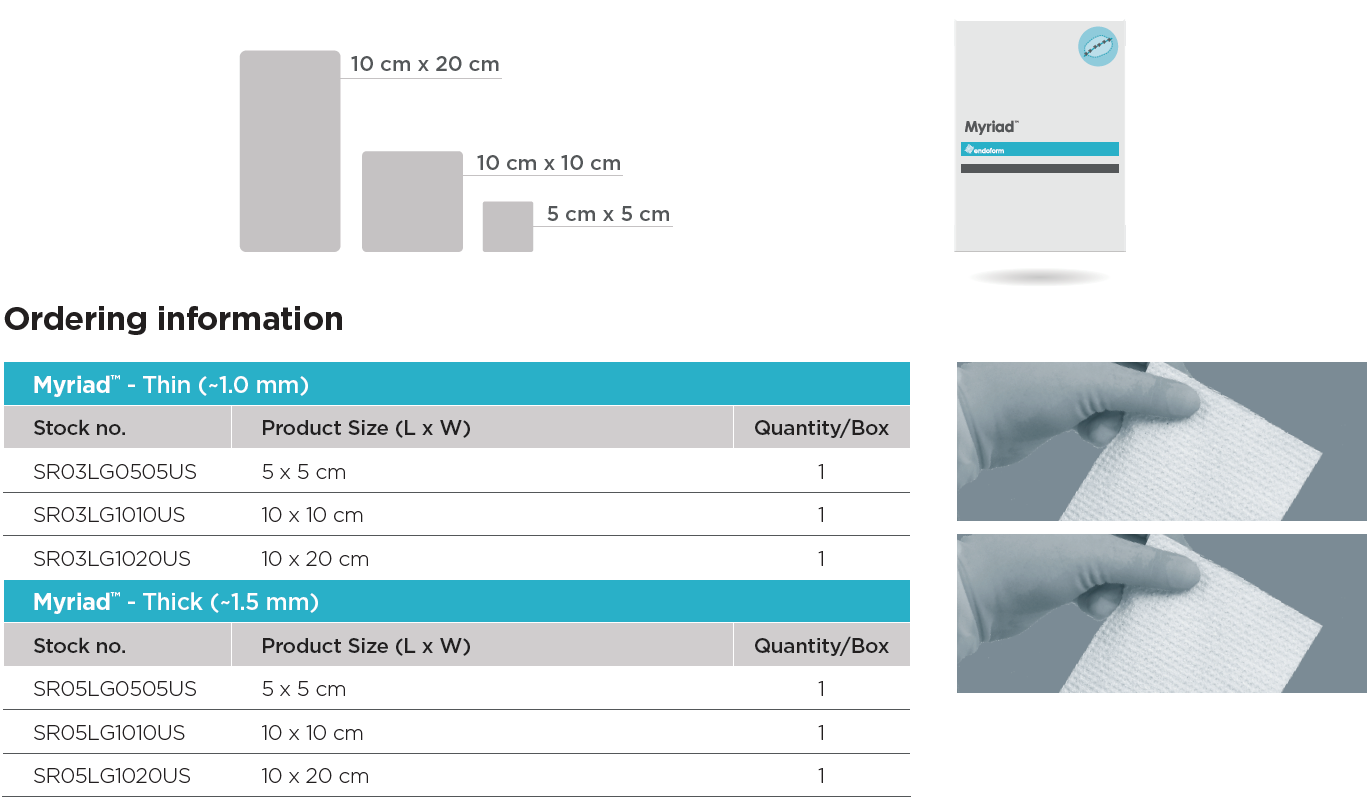
Myriad™ Soft Tissue Bioscaffold is an engineered extracellular matrix (ECM) for soft tissue repair, reinforcement and complex wounds. Myriad™ has been specifically designed to help maximize tissue repair while providing versatility and adaptability across a wide range of surgical applications.
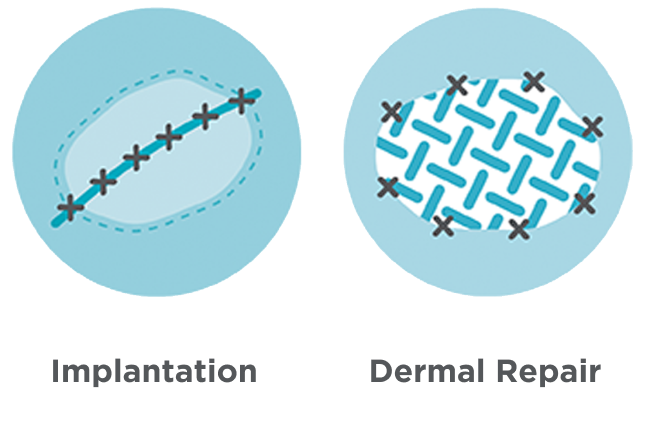
Myriad™ is designed to be easily customizable for a wide range of anatomical sites and surgical needs and can be used in both implantation and dermal repair.

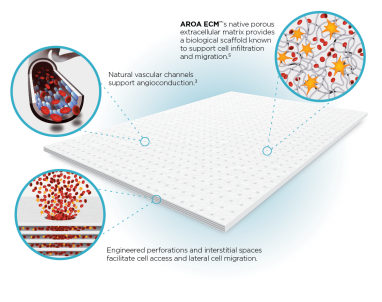
Myriad™ devices contain the natural porous structure of AROA ECM™, engineered with interstitial perforations to enable cell infiltration to facilitate rapid healing
Fast absorption of blood & cells accelerates host cell infiltrationThe rapid absorption of blood and blood components forms a reservoir of biologically important cells and cell components to help kick-start the tissue repair process.(1-4) Fibroblast, endothelial and immune cells infiltrate the entire matrix, build new tissue and over time Myriad™ is completely remodeled by the patient’s own tissue.(3, 4)
Delivers biology known to be important in healingMyriad™ retains the authentic structure and complexity of natural tissue ECM and provides biological cues to aid the repair process.(5) Myriad™ contains more than 150 ECM proteins, including collagen and other secondary molecules that exist in tissue and aid the healing process.(6)
Contains residual vascular channelsAngioconduction is the structural effect of vascular channels on endothelial cells to support blood vessel development. Myriad™ promotes angioconduction via the residual vascular channels within the Aroa ECM™ source biomaterial, helping to facilitate the rapid establishment of a dense capillary network.(7, 8)
Myriad™ is indicated for implantation to reinforce soft tissue where weakness exists in patients requiring soft tissue repair or reinforcement in plastic and reconstructive surgery, or for management of the following wounds:
Desvigne, M. N., K. Bauer, K. Holifield, K. Day, D. Gilmore and A. L. Wardman (2020). “Case Report: Surgical Closure of Chronic Soft Tissue Defects Using Extracellular Matrix Graft Augmented Tissue Flaps.” Frontiers in Surgery 7(173).
Desvigne, M. N., K. Bauer, K. Holifield, K. Day, D. Gilmore and A. L. Wardman (2020). “Case Report: Surgical Closure of Chronic Soft Tissue Defects Using Extracellular Matrix Graft Augmented Tissue Flaps.” Frontiers in Surgery 7(173).
Bohn, G. A. (2020). “Using Ovine Extracellular Matrix in Difficult to Close Excisions of Common Skin Cancer: an Evolving New Technique.” Surg Technol Int 37: 49-53.
Bohn, G. A. and A. E. Chaffin (2020). “Extracellular matrix graft for reconstruction over exposed structures: a pilot case series.” J Wound Care 29(12): 742-749.
Simcock, J., & May, B. C. (2013). Ovine forestomach matrix as a substrate for single-stage split-thickness graft reconstruction. Eplasty, 13, e58.
Wahab, N., Lapucha, M., and C. Chauhan (2020). Ovine Forestomach Matrix is Efficacious in the Closure of Significant Post Surgical Undermining. Symposium on Advanced Wound Care – Spring, Virtual Conference.
Chaffin, A. E., and M. Buckley (2020). Clinical Evaluation of an Extracellular Matrix Graft for the Surgical Management of Hurley Grade III Hidradenitis Suppurativa. Symposium of Advanced Wound Care, Virtual Conference.
Chaffin, A. E. and M.-C. Buckley (2020). Surgical Management of Hurley Stage III Hidradenitis Suppurativa Using ECM Graft. Symposium on Hidradenitis Suppurativa Advances, 5th Annual SHSA, October 9-11 2020, Virtual.
Geiger, J. E. J. and J. Skwarczyk (2020). Extracellular matrix-based regeneration over exposed bone and tendon: A prospective case series. DFCon 20 Conference, Oct 9-10 2020, Virtual.
Chaffin, A. E., A. M. Aballay, G. A. Bohn, P. M. Glat, M. N. Desvigne and B. C. H. May (2019). Multi-Centre Clinical Evaluation of a Cell Conductive Extracellular Matrix Surgical Mesh in Plastics and Reconstructive Surgery – A Case Series. 41st Annual Boswick Burn & Wound Symposium, Wailea Beach, Maui, HI.
Chaffin, A. E., A. M. Aballay, G. A. Bohn, P. M. Glat, M. N. Desvigne and B. C. May (2019). Multi-Centre Clinical Evaluation of a Cell Conductive Extracellular Matrix Surgical Mesh in Plastics and Reconstructive Surgery – A Case Series. SESPRS 62nd Annual Scientific Meeting, Naples, Florida.
Ferreras, D. T. and R. L. Crump (2019). Use of a High-Density ECM for the Management of Deep Diabetic Foot Ulcers: A Case Series. Symposium for Advanced Wound Care – Fall, Las Vegas, NV.
Chaffin, A. E. and G. Bohn (2019). Clinical Evaluation of an Extracellular Matrix Surgical Graft for Reconstructive Surgery over Exposed Bone or Tendon. Symposium for Advanced Wound Care – Fall, Las Vegas, NV.
Yüksek Kapasiteli Siparişleriniz İçin İletişime Geçiniz


